MIXR Partnership Seeks to Expand Its Research and Outreach Efforts
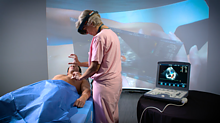
A unique multi-institutional partnership that focuses on extended reality (XR) technologies for clinical procedures and medical training is ramping up efforts to expand its research, outreach and impact.
The Center for Medical Innovations in Extended Reality (MIXR)—launched in 2022 with a $5 million award from the National Science Foundation and industry partners—is currently soliciting broader participation from private sector companies interested in developing XR-driven hardware and software tools that can be used to save lives and improve medical outcomes.
XR is the umbrella term used for technology based in virtual, augmented and blended realities. Prototype testing of XR technology used for diagnostic imaging, surgical training and improved bedside procedures is already underway, with other immersive tools used for virtual trainings and assessments of medical students.
“We have our technical and administrative infrastructure in place and are moving forward on several fronts that we believe will offer medical professionals new ways to access and interpret vital information used in clinical settings,” says Amitabh Varshney, professor and dean of the College of Computer, Mathematical, and Natural Sciences at the University of Maryland. 
(Pictured from left to right) Varshney is the lead-site principal investigator for MIXR, joined by partner-site PIs Sarah Murthi, M.D., a professor of surgery at the University of Maryland School of Medicine; and Rishindra Reddy, M.D., M.B.A., a professor of thoracic surgery at the University of Michigan.
In collaboration with the U.S. Food & Drug Administration (FDA) and current industry partners Sony and Microsoft, MIXR is currently honing in on two research areas: characterizing cybersickness in users that experience it when using virtual environments; and markerless registration, used to streamline visual tracking systems used in most XR technologies. This year, MIXR has added industry partners MediView and Cook Advanced Technologies to the center, and looks forward to welcoming the U.S. Air Force this month.
Sony serves as the industry mentor for the cybersickness project, with the research being led in Varshney’s lab with his graduate students.
Microsoft is working with computer scientists and clinicians involved in MIXR to advance markerless registration. “The goal of that project is to place virtual 3D objects in the physical environment—depending on the environment's real features rather than identifying markers—thus eliminating the need for object tracking systems," Reddy explained.
Any new XR technologies must first be approved by the FDA before they are implemented in a clinical setting.
Ed Margerrison, director of science and engineering for the FDA, says that there is a big push to make the regulatory processes for XR technologies designed for medical purposes more efficient, ensuring that safe, effective and innovative clinical solutions make it to patients as soon as possible.
Margerrison emphasized that his agency’s participation in MIXR is a good opportunity for federal regulatory scientists “to learn about these new technologies and help move them forward in a responsible way.”
Varshney and Murthi were recently featured in the Washington Post discussing the value of VR and AR for professional training and medicine, and IEEE Pulse magazine published an article highlighting the center’s launch and research activities.
Barbara Brawn, managing director of MIXR, says there are multiple opportunities ahead for the center to explore, including the use of artificial intelligence and machine learning for predictive modeling and assessment of medical imagery.
“We anticipate that MIXR will take a leadership role in the development of safe and effective extended-reality technologies for health care, whether that be for diagnosis, treatment, medical training or patient education,” she says.
Corporations or startups interested in partnering with MIXR should contact Brawn at: [email protected]
